
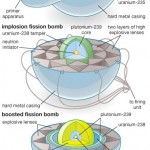
The release and diffusion of tritium and helium produced by the fission of lithium can take place within ceramics referred to as breeder ceramics. Tritium is most often produced in nuclear reactors by neutron activation of lithium-6.

The low energy of tritium's radiation makes it difficult to detect tritium-labeled compounds except by using liquid scintillation counting. The unusually low energy released in the tritium beta decay makes the decay (along with that of rhenium-187) appropriate for absolute neutrino mass measurements in the laboratory (the most recent such experiment being KATRIN). Because of their low energy compared to other beta particles, the amount of bremsstrahlung generated is also lower. Beta particles from tritium can penetrate only about 6.0 millimetres (0.24 in) of air, and they are incapable of passing through the dead outermost layer of human skin. The electron's kinetic energy varies, with an average of 5.7 keV, while the remaining energy is carried off by the nearly undetectable electron antineutrino. It decays into helium-3 by beta-minus decay as per this nuclear equation:Īnd it releases 18.6 keV of energy in the process. This implies that, per year, approximately 5.5% of a given sample of tritium will decay. While tritium has several slightly different experimentally determined values of its half-life, the National Institute of Standards and Technology lists 4,500 ± 8 days ( 12.32 ± 0.02 years). Willard Libby recognized in 1954 that tritium could be used for radiometric dating of water and wine.
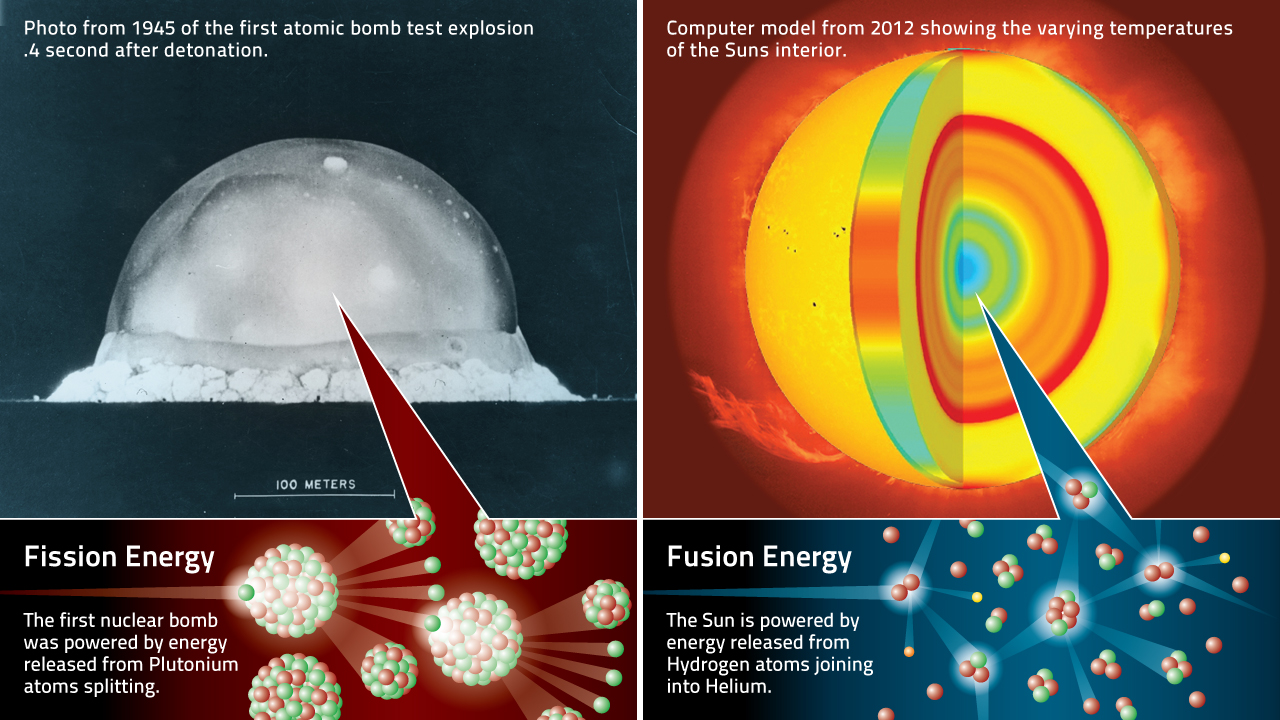
Their experiment could not isolate tritium, which was first accomplished in 1939 by Luis Alvarez and Robert Cornog, who also realized tritium's radioactivity. Deuterium is another isotope of hydrogen, which occurs naturally with an abundance of 0.015%. Tritium was first detected in 1934 by Ernest Rutherford, Mark Oliphant and Paul Harteck after bombarding deuterium with deuterons (a proton and neutron, comprising a deuterium nucleus). Tritium is also used as a nuclear fusion fuel, along with more abundant deuterium, in tokamak reactors and in hydrogen bombs. It is used in a medical and scientific setting as a radioactive tracer. Tritium is used as the energy source in radioluminescent lights for watches, gun sights, numerous instruments and tools, and even novelty items such as self-illuminating key chains. It can be produced artificially by irradiation of lithium metal or lithium-bearing ceramic pebbles in a nuclear reactor and is a low-abundance byproduct in normal operations of nuclear reactors. The atmosphere has only trace amounts, formed by the interaction of its gases with cosmic rays. Naturally occurring tritium is extremely rare on Earth. The nucleus of tritium (t, sometimes called a triton) contains one proton and two neutrons, whereas the nucleus of the common isotope hydrogen-1 ( protium) contains one proton and zero neutrons, and that of hydrogen-2 ( deuterium) contains one proton and one neutron. Tritium (from Ancient Greek τρίτος (trítos) 'third') or hydrogen-3 (symbol T or 3H) is a rare and radioactive isotope of hydrogen with a half-life of about 12 years.


 0 kommentar(er)
0 kommentar(er)
